
The Reagan-Udall Foundation for the FDA (the Foundation) is pleased to announced the release of “Transforming Animal Health In The U.S. For The 21st Century,” a report prepared by an Expert Panel tasked with identifying holistic approaches to address opportunities and challenges in animal health and veterinary medicine. At the request of the Food and Drug Administration’s Center for Veterinary Medicine (FDA-CVM), the Foundation convened an Expert Panel to conduct an analysis of the U.S. animal health and veterinary industry.
Expert Panel members are:
- Lisa A. Tell, DVM, DACZM, DABVP (Avian) - Chair
- Caitlin Boon, PhD
- Sathya Chinnadurai, DVM, DACZM, DACVAA, CCRP
- Virginia Fajt, DVM, PhD, DAVCVP
- Randall Gnatt, JD
- Michelle Kromm, DVM, MPH, MAM, DACPV
- Katelyn McCullock, MS
- Kurt Schrader, DVM
Process
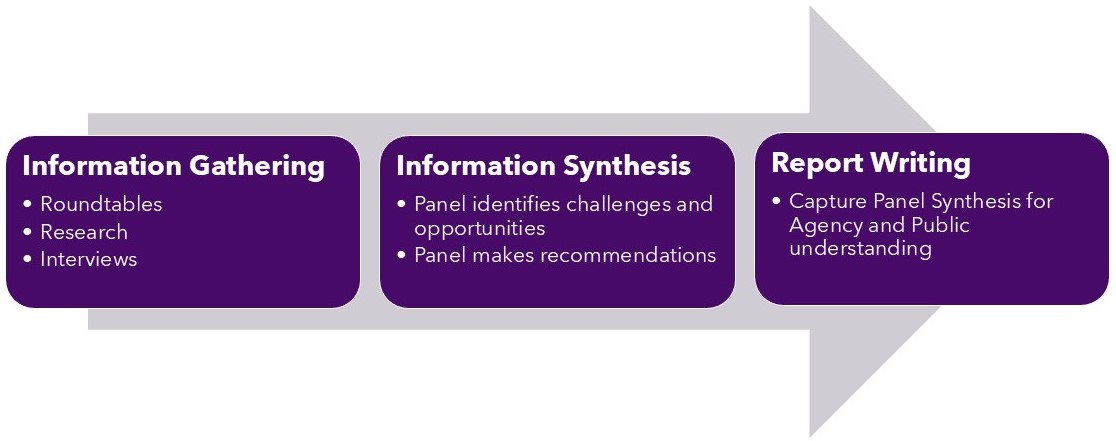
Number of Meetings
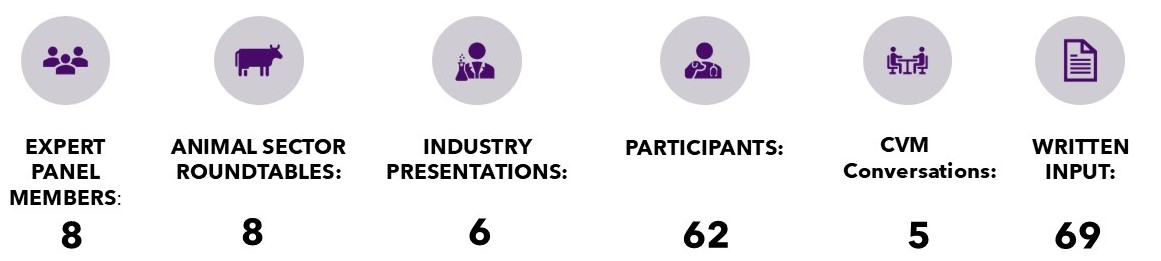
The Expert Panel held numerous conversations with experts and invited written submissions; ultimately finding the current laws and regulatory authorities governing animal health and nutrition in need of updating. Cumbersome requirements, poor communication, and outdated regulatory pathways have made the U.S. less competitive in the global race for new animal drugs, therapeutic treatments, and animal nutritional ingredients. This poses a challenge not just for U.S. competitiveness, but also for our food supply.
While this report is inherently FDA-centric, the interconnectedness of the animal health ecosystem will require multiple government agencies and Congress to improve. Expert Panel Chair Lisa A. Tell, DVM, DACZM, DABVP (Avian), observed “While chairing this Expert Panel, it was readily apparent to me that advancing animal health into the 21st century is going to be a complicated process that will require dynamic engagement. It is going to require agencies and stakeholders to work collaboratively in order to start making impactful differences. This synergistic approach will be especially important if we want to continue to be global leaders in agriculture, animal health, and animal welfare.”
The Expert Panel’s findings and recommendations are intended to empower the animal owner and veterinary sector (including government and the private sector) to fill gaps and unmet needs in animal health (therapeutic interventions and food/feed.
This project is supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of an award of $325,000 in federal funds (100% of the project). The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA, HHS, or the U.S. Government. For more information, please visit FDA.gov.
