SUD: Nicotine, Cannabis, and Alcohol Demand Forecasting
Clinical Practice Guidelines PUE/MOUD
Opioid Disposal Online Controlled Substances Summit
Supply Chain for Prescription Medications Xylazine
Psychedelic Clinical Study Design Prescription Stimulant Availability
Buprenorphine Understanding Fatal Overdose
Stimulant Use Disorder Treatment Harm Reduction Roundtables
Individual & Provider Perspectives Naloxone Access Naloxone Economic Impact Payor Perspectives
The United States is facing an overdose crisis. Over 107,000 Americans died of drug overdoses in 2021, according to the Centers for Disease Control and Prevention’s provisional estimates. According to the Department of Health and Human Services (HHS), the overdose crisis has evolved over time and is now  largely characterized by deaths involving illicitly manufactured synthetic opioids, including fentanyl, and, increasingly, stimulants. Since 1999, the rate of overdose deaths has increased by more than 250%. In response to these changes, HHS launched its Overdose Prevention Strategy to focus on primary prevention, harm reduction, evidence-based treatment, and recovery support.
largely characterized by deaths involving illicitly manufactured synthetic opioids, including fentanyl, and, increasingly, stimulants. Since 1999, the rate of overdose deaths has increased by more than 250%. In response to these changes, HHS launched its Overdose Prevention Strategy to focus on primary prevention, harm reduction, evidence-based treatment, and recovery support.
The Reagan-Udall Foundation for the FDA (FDA Foundation) supports the U.S. Food and Drug Administration’s work to develop strategies for the prevention, treatment, and management of substance use disorders by engaging with stakeholders in the health, regulatory, industry, caregiver and advocacy communities to identify and address critical gaps across the SUD treatment system.

The Foundation collaborated with the FDA to explore reasons for use, attitudes toward cessation, and unmet treatment option needs for individuals who excessively use nicotine, cannabis, and alcohol or have been diagnosed with a related substance use disorder. Learnings included noted similarities in behaviors and are noted in the summaries on nicotine and cannabis use.
Each year, the Food and Drug Administration (FDA) submits an estimate of medical, scientific, and reserve stock needs for Schedule I and II substances to the Drug Enforcement Administration (DEA). In turn, DEA uses these estimates, along with data from other sources, to set an overall quota or limit for these substances, as well as individual quotas for manufacturers of these substances. In August 2025 the Foundation, in collaboration with the FDA, hosted a hybrid public meeting on "Demand Forecasting for Controlled Substances." This meeting included exploration of the complex factors that are considered when estimating the medical, scientific, and reserve stock needs for Schedule I and II substances, such as opioids and stimulants. Speakers included representatives from government agencies, the private sector, and academic researchers. Click here to read the summary report, watch the meeting recording, and view meeting materials.
Developing clinical practice guidelines (CPGs) for controlled substances requires balancing scientific rigor, clinical practicality, and patient-centered care. In June 2025, the Reagan-Udall Foundation for the FDA, in collaboration with FDA, convened five FDA-funded research teams, federal partners, and guideline developers to explore how to strengthen these efforts—from addressing evidence gaps to improving stakeholder engagement, dissemination, and implementation. The report summarizes key insights on developing clinical practice guidelines involving controlled substances and explores strategies for addressing evidence limitations, engaging stakeholders, and improving communication and implementation.
Medications for opioid use disorder can be lifesaving for those who need them, but deadly for young children—even in small amounts. To better understand the risk of PUE to these medications, the Foundation collaborated with FDA to identify knowledge gaps in current storage and emergency response information provided to parents/caregivers/household members who take MOUD and live in households with young children. Through a thorough scan of existing literature on the topic, discussions with experts, interviews/conversations, and online polling of individuals with and without lived experience, we developed message frameworks and strategies to reduce risk of PUE and poisoning, without adversely affecting patient access or contributing to stigma.
The Foundation collaborated with FDA to explore the current landscape and potential opportunities for safe disposal of unused and expired opioid medications. The project focused on understanding consumer perspectives and needs related to opioid disposal.
The Food and Drug Administration (FDA) and the Reagan-Udall Foundation hosted the sixth Online Controlled Substances Summit on September 11, 2025. The Summit explored emerging trends in the online ecosystem, including youth behaviors and platform usage, the human impact of uncontrolled access, and opportunities for intervention and risk reduction. Sessions featured leadership from the FDA, researchers and educators, families who bravely shared first-hand experiences, digital technology companies, and international partners. The sixth event of its kind, the Summit brought the community together to share insights, identify evolving threats and discuss innovative approaches to address this ongoing and complex public health concern.
The Reagan-Udall Foundation for the FDA, in collaboration with the U.S. Food and Drug Administration, hosted a hybrid public meeting titled Understanding Current Use of Ketamine for Emerging Areas of Therapeutic Interest. The meeting brought together clinicians, researchers, patient advocates, federal partners, and professional organizations to examine the evolving use of ketamine in clinical settings.
Discussions focused on emerging therapeutic applications, safety considerations, and the growing online promotion and access to ketamine. Presenters also reviewed the science behind ketamine’s pharmacology—including its approved use as an anesthetic and its potential antidepressant effects—and considered regulatory pathways, such as the supplemental new drug application (sNDA), for expanding approved indications. Insights from esketamine (Spravato®) development were explored as a potential model for future regulatory review.
The Foundation hosted a public meeting titled "Advancing Treatments for Post- Traumatic Stress Disorder," in alignment with the FDA's ongoing efforts to better understand the medical needs surrounding PTSD with a goal of facilitating treatment development. This meeting included a panel discussion with federal partners exploring efforts to accelerate treatment development for PTSD, including the potential role of psychedelic drugs. Following the panel, stakeholders were invited to share their perspectives, both in-person and virtually. A diverse range of voices contributed, including individuals with lived PTSD experience, their families, patients, advocates, researchers, scientists, and drug developers.
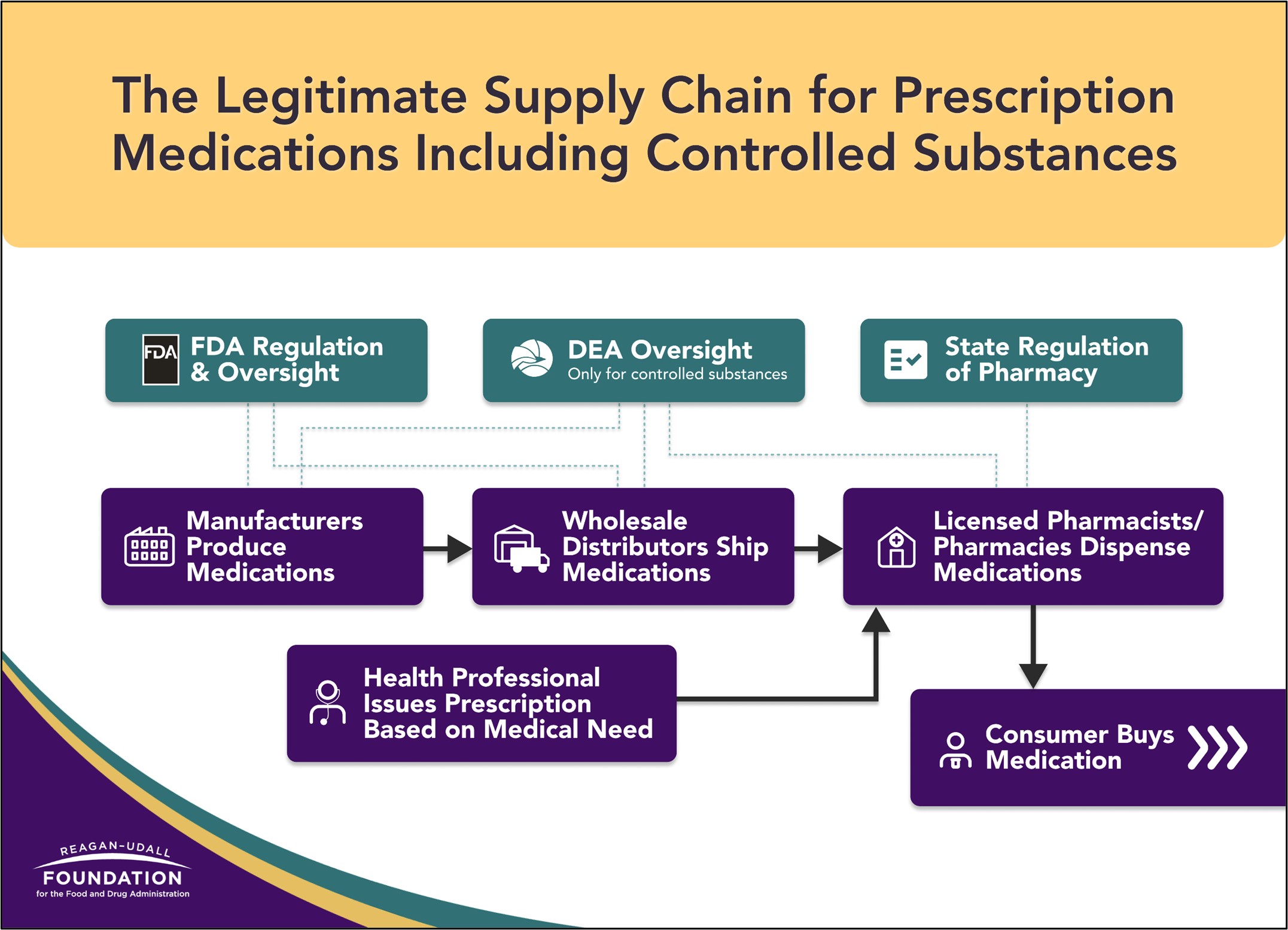
As people turn toward telehealth, accessing care through video calls or app-based services, they are also turning online to access medications. Purchasing controlled substances and other prescription medications online may occur through licensed pharmacies, but also via illegal online pharmacies or non-licensed sellers on websites, social media or apps—often without the consumer even realizing. Differentiating between legitimate and nonlegitimate sources of prescription medications can be tricky. This infographic illustrates the legitimate supply chain for prescription medications.
The Foundation partnered with the Food and Drug Administration (FDA) to host a public meeting on "Mitigating Risks from Human Xylazine Exposure" that explored real-world experiences and scientific evidence on emerging data trends. The meeting also examined concrete strategies for drug development and clinical research that directly support the mitigation and reduction of risks associated with human exposure to xylazine. Presenters and discussants included clinical and scientific experts, community and harm reduction organizations, academic researchers, and federal partners. The Foundation produced a report from the meeting that outlines actions needed to address xylazine exposure and the larger overdose crisis.
In June 2023, FDA issued its first psychedelics draft guidance for industry, Psychedelic Drugs: Considerations for Clinical Investigations, to provide general considerations to sponsors developing psychedelic drugs for treatment of medical conditions. While the guidance highlights some considerations for designing clinical trials with psychedelics to optimize the interpretability of results, many questions remain about the most appropriate way to address these challenges. The FDA Foundation convened “Advancing Psychedelic Clinical Study Design,” a public meeting to discuss the experience of scientists working with psychedelics in FDA-authorized clinical studies and drug development, considerations for psychedelics in clinical trial designs, and perspectives and current research in psychedelic clinical trials. The meeting also provided an overview of Psychedelic Drugs: Considerations for Clinical Investigations.
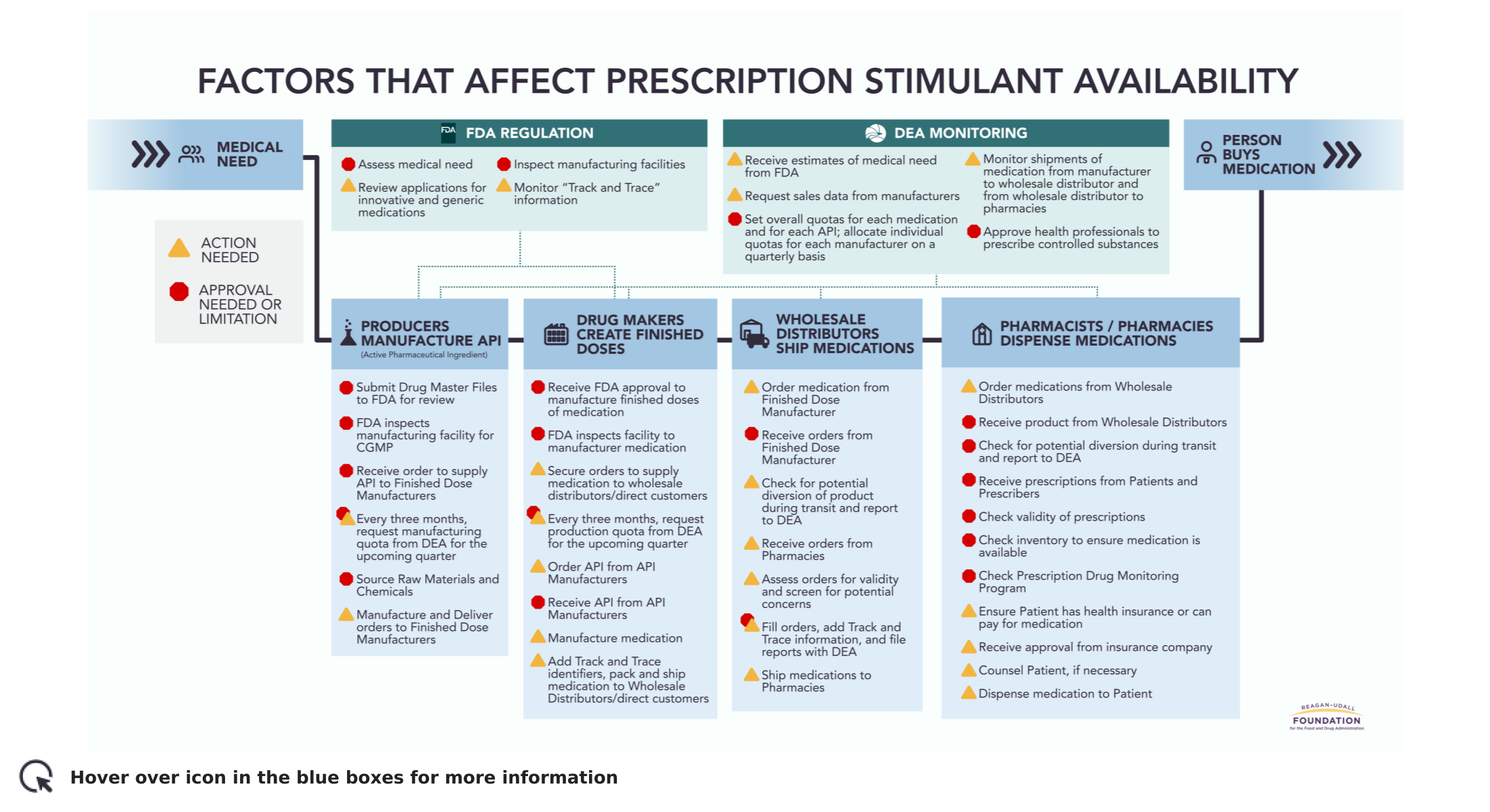
In recent months, families and individuals have reported increased challenges in accessing prescribed stimulant medications such as those used to treat Attention-Deficit/Hyperactivity Disorder (ADHD). The Foundation, in partnership with the FDA, has embarked on a new project to illustrate the factors that affect prescription stimulant availability.
The pathway to access these medications, classified as controlled substances, may seem straightforward with a relatively simple supply chain. In reality, the path of a prescription stimulant—from approval through manufacturing, distribution, and dispensing to the patient—has a number of challenging steps and requirements, any of which may slow down or interrupt the movement and accessibility of the medication. This preliminary image presents an illustrative but not exhaustive view of that complexity.
Buprenorphine is a safe and effective prescription medication used for the treatment of Opioid Use Disorder (OUD). Initiation of buprenorphine takes careful planning: patients must already be experiencing mild to moderate withdrawal symptoms or risk precipitated withdrawal. An increase in fentanyl in the illicit drug supply further complicates buprenorphine initiation and maintenance adding to the access challenge.
The Foundation, in collaboration with FDA and the Substance Abuse and Mental Health Services Administration, held a two-part virtual public meeting on “Considerations for Buprenorphine Initiation and Maintenance Care” to support efforts to develop products and approaches to treat OUD.
This report summarizes the discussions and presentations which explored solutions, innovative public health interventions and needed investments in research and product development to spur progress.
Drug overdose persists as a major public health issue in the U.S. Synthetic opioids, such as fentanyl and its analogs, are the primary driver of the increase in overdose deaths.
In March 2023, the FDA Foundation, in partnership with the Food and Drug Administration (FDA) and the Substance Abuse and Mental Health Services Administration (SAMHSA), hosted a virtual public workshop titled “Understanding Fatal Overdoses to Inform Product Development and Public Health Interventions to Manage Overdose” to examine the evolving context surrounding fatal overdoses and strategies to better manage overdose moving forward.
This summary report explores solutions and addresses regulatory considerations for future action and innovation.
The FDA Foundation, in collaboration with FDA and the National Institute on Drug Abuse (NIDA), hosted a virtual public workshop to discuss a practical research agenda toward treatment development for stimulant use disorder.
Meeting participants responded to a proposed practical research agenda that focuses on innovation in clinical trial design and candidate endpoints for the evaluation of potential treatments for stimulant use disorder.

Individuals inherently deserve services that promote health, regardless of whether they use drugs. Harm reduction is a set of proactive and evidence-based approaches that reduce the negative personal and public health impact associated with the use of alcohol and other drugs.
The FDA Foundation, working with FDA and several operating divisions within HHS, held two roundtables exploring community and clinical perspectives on fentanyl drug checking and screening. Community participants discussed their experiences using fentanyl test strips (FTS) and other drug checking methods, and clinician and researcher participants shared their perspectives on both clinical fentanyl testing and distribution of FTS to patients.
The FDA Foundation partnered with FDA’s Center for Drug Evaluation and Research to gain a deeper understanding of individuals’ experiences with substance use disorders (SUDs) – and specifically with SUD treatment. Our approach was to track individual treatment journeys to identify the specific challenges they faced and the supports they found helpful. Our goal is to use this ethnographic research to identify potential system improvements and engagement opportunities that can help inform FDA’s work in the prevention and treatment of SUDs. For this project, we looked at two specific issues: experiences exploring or using medication for opioid use disorder (MOUD), and trajectories of treatment for individuals who have pursued SUD treatment more than once.
Individuals exploring the use of medication to treat SUDs do not travel their paths alone. They are connected to networks of providers, peers, and family members who may have their own experiences, opinions, training, and expertise. Mapping individuals’ experiences led us to also map the experiences – and potential influences – of prescribing clinicians, non-prescribing clinicians such as counselors and therapists, and pharmacists. Individuals and these provider groups demonstrated generally positive views of MOUD and SUD treatment in general.
The Foundation teamed with FDA to host this virtual public meeting addressing some of the most frequently asked questions about access to naloxone, an antidote to opioid overdose.
Harm reduction specialists, physicians, pharmacists, and regulators explored the current landscape of naloxone availability, examined perceived barriers to naloxone access, and addressed questions posed by various groups on this subject. We then opened mics for nearly an hour of public comment to hear first hand from communities.
The Naloxone Economic View Summary Report explores the current distribution of naloxone in the U.S. and the potential economic impacts of a change in the prescription-only status of naloxone. The Foundation, in collaboration with FDA, analyzed data to estimate the amount of naloxone distributed in 2021. The report also includes findings from our expert roundtable with the payor community focused on understanding how a shift is the prescription status of naloxone might impact costs and consumer access.
The FDA Foundation convened, in conjunction with FDA’s Center for Drug Evaluation and Research, a roundtable on “Payor Perspectives on Substance Use Disorder Treatment.” The purpose of the roundtable was to hear key stakeholders’ perspectives on health insurance coverage of pharmacological treatments for SUDs, including how FDA approvals and labeling affect payors and insurance coverage.
Patient advocates, clinicians, payors, health economists, and federal leaders all participated. Attendees discussed the data framework insurance companies utilize in making coverage decisions for patients receiving substance use disorder treatments. The convening additionally generated real-world insight into how regulatory decisions are applied in ways that impact people’s daily lives.