Accelerated Approval Program
30 Years On: Insights and Experiences
March 11, 2022 | 1-4 PM eastern
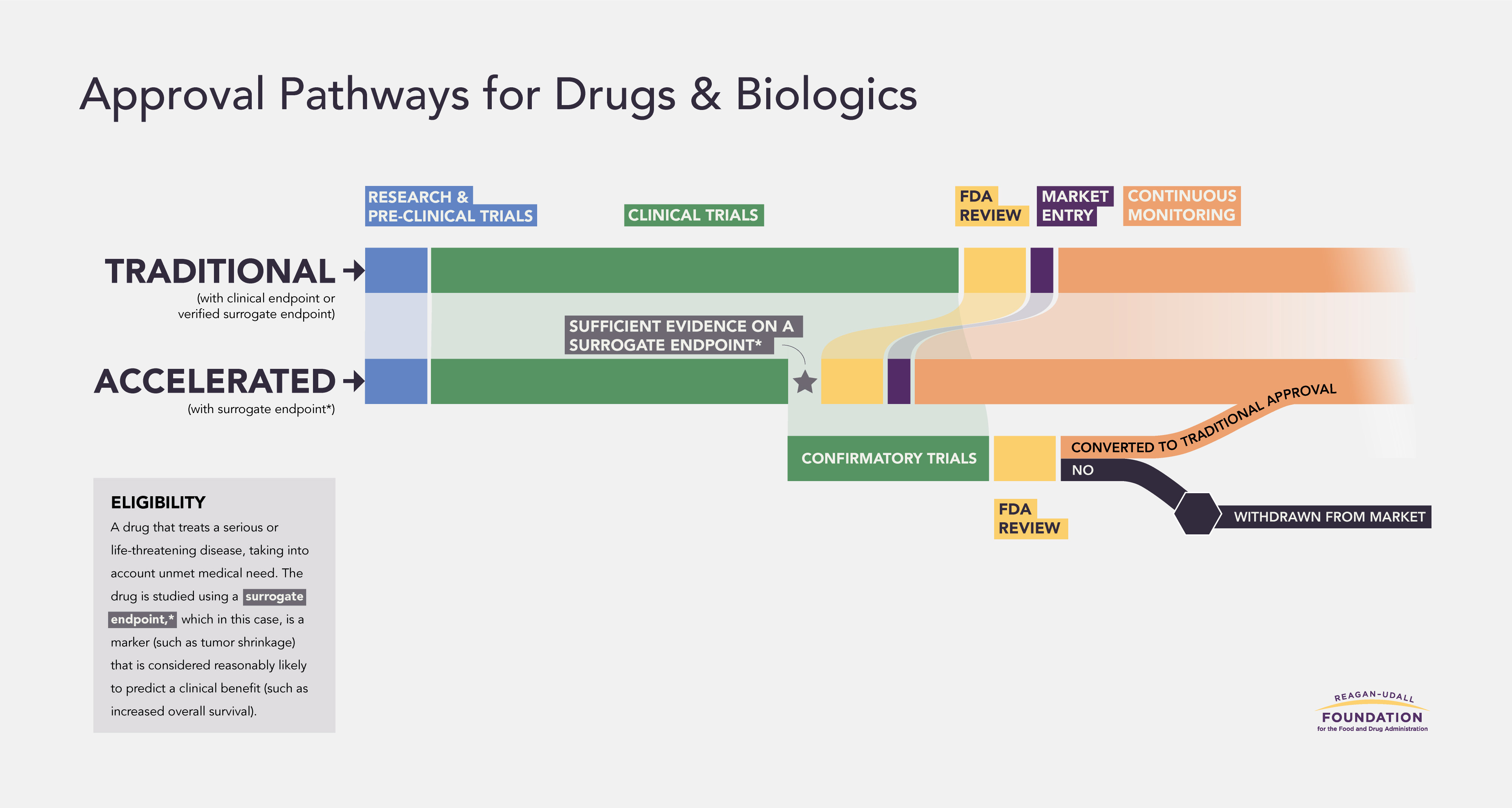 The Accelerated Approval Pathway was created by Congress to allow patients with a serious or life-threatening disease earlier access to a potentially important treatment. At this virtual public meeting we explored the insights and lessons learned in the 30 years since its inception.
The Accelerated Approval Pathway was created by Congress to allow patients with a serious or life-threatening disease earlier access to a potentially important treatment. At this virtual public meeting we explored the insights and lessons learned in the 30 years since its inception.
View the recorded event and read meeting materials below.
 Read the transcript Read the transcript |
 View the slide deck View the slide deck |
Agenda
Moderated by Susan C. Winckler, RPh, Esq.
CEO, Reagan-Udall Foundation for the FDA
| 1 PM | Welcome & Opening Remarks |
|---|---|
| 1:05 |
Accelerated Approval 1992-2022
|
| 2:05 |
Patient Perspective Panel
|
| 3:00 |
Fireside Chat Sharing Patient Advocacy, Provider, Industry, and Payer Perspectives
|
| 3:55 | Closing Remarks/Adjourn |
Speaker & Moderator Biographies
