Exploring the Potential for a Public-Private Partnership to Support the Tracking and Monitoring of Antimicrobial Use in Food-Producing Animals
Virtual Public Forum
Tuesday, June 14, 2022
1-3pm Eastern Time
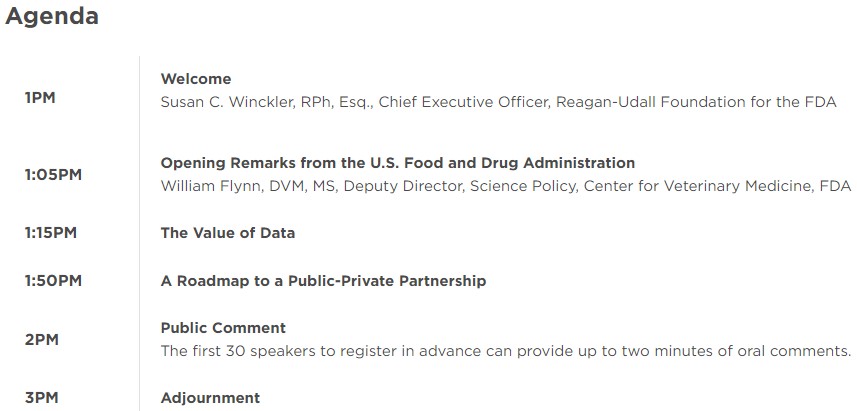
Through a cooperative agreement with the FDA's Center for Veterinary Medicine (CVM) we've been exploring the development of a public-private partnership for collecting and analyzing real-world data regarding antimicrobial use (AMU) in food-producing animals. Over the last several months, we have convened and facilitated a series of working sessions with stakeholders to gather input on the benefits, costs, and challenges of creating and maintaining a voluntary AMU data repository that could enhance understanding of how antimicrobials are used in animals and support antimicrobial stewardship. These discussions with stakeholders from animal agriculture, veterinary, public health organizations, and other key representatives were summarized in a report entitled, “Exploring the Potential for A Public-Private Partnership to Support the Tracking and Monitoring of Antimicrobial Use in Food-Producing Animals.”
We held a virtual public forum on June 14, 2022 to discuss the value of establishing a public-private partnership to collect and analyze antimicrobial use data from food-producing animals. Watch the event below.
Meeting Materials
Funding Disclosure: This project is supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of an award of $65,329 in federal funds (100% of the project). The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA, HHS, or the U.S. Government. For more information, please visit FDA.gov.