- About
-
Projects
-
Column 1
- Advancing Regulatory Science
- Animal Health & Veterinary Medicine
- Expanded Access
- FDA Patient Listening Sessions
-
Column 2
- Food & Nutrition
- Improving Access to FDA Information
- Research
- Substance Use Disorders
-
Column 1
- News and Events
- Expanded Access eRequest
The FDA Foundation Research Program works to accelerate and improve the availability of safe and effective medical products.
The Expanded Access Navigator, or EA Navigator, represents a unique partnership between the Reagan-Udall Foundation for the FDA, patient advocacy organizations, the pharmaceutical industry, and the federal government to provide clear, digestible information on single-patient EA.
The Reagan-Udall Foundation for the FDA convened an invitation-only roundtable conversation with industry stakeholders and patient representatives to discuss challenges in rare disease drug development and seek ways to enhance the efficiency and agility of generating evidence for regulatory review of rare disease medicines. We are preparing a topline learnings summary report capturing the insights from this roundtable that will be publicly available.
Alnylam, argenx, BridgeBio, Jazz Pharmaceuticals, Lilly, and Stoke Therapeutics provided funding for…
The Reagan-Udall Foundation for the FDA facilitated an effort to explore actionable strategies for improving the clarity, consistency, and efficiency of oncology multi-regional clinical trials (MRCTs). The project addressed challenges to timely site activation and patient enrollment, opportunities to address regulatory and operational barriers through technology and trial modernization (including U.S.
The Foundation convened a series of invitation-based roundtable conversations to discuss ways to support use of the updated “healthy” claim and, explore ways to support use of and a potential symbol.
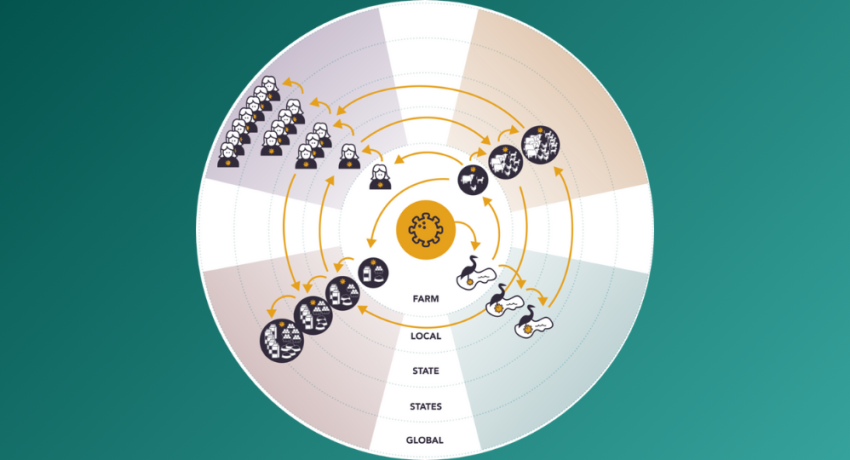
At FDA's request, the Foundation identified opportunities for the agency to address challenges at the intersection of human and animal health sectors, including those in the ongoing response to the current H5N1 (Avian influenza) outbreak.
The Reagan-Udall Foundation for the FDA is leading a stakeholder dialogue process to explore new strategies for produce safety and the development of a collaborative entity to drive progress.
The Reagan-Udall Foundation for the FDA was requested by FDA's Center for Veterinary Medicine to identify holistic approaches to address opportunities and challenges in animal health and veterinary medicine.
The project set out to discover what patients, caregivers, healthcare professionals, and researchers want to know about FDA-regulated medical products (drug and biologics); where they seek this information; what FDA data are publicly available for external sources to utilize in generating query responses.
As announced on July 19, 2022, the Reagan-Udall Foundation facilitated, via two Independent Expert Panels, operational evaluations of FDA’s human foods and tobacco programs. Each evaluation yielded a report with operational recommendations to the FDA: one for human foods and the other for tobacco. Each evaluation, and therefore report delivery, was on its own 60-business-day timeline. Both reports were delivered to the FDA Commissioner and made available to the public.
Learn how the Reagan-Udall Foundation for the FDA supports strategies to prevent, treat, and manage substance use disorders by engaging stakeholders and addressing critical gaps in the SUD treatment system.
The Reagan-Udall Foundation for the FDA is partnering with the FDA's Center for Veterinary Medicine (CVM) to explore the development of a public-private partnership framework for collecting and analyzing real-world data regarding antimicrobial use (AMU) in food-producing animals.
The Reagan-Udall Foundation for the FDA's work supports FDA’s advancement of the Nutrition Innovation Strategy, promotes actions to reduce preventable death and disease related to poor nutrition. The Foundation is engaging with stakeholders -- including regulators, consumers, and industry -- to further food system strategies that foster industry innovation and change in consumer behavior.
Our COVID-19 Hub includes a Directory of Companies Developing COVID-19 Therapies, a searchable listing of relevant Clinical Trials and Expanded Access Opportunities culled from ClinicalTrials.gov, and links to the latest FDA, CDC, and NIH updates. In addition to medical interventions, the COVID-19 Hub also links to critical FDA resources for consumers and industry focused on food and veterinary safety.
FDA Patient Listening Sessions are one way patient communities and FDA staff connect about the experiences of living with specific diseases and conditions. The Reagan-Udall Foundation works closely with the Patient Affairs team in FDA’s Office of Clinical Policy and Programs/Office of the Commissioner to conduct these small, informal discussions. Patients, caregivers, and advocates provide personal insight into the day-to-day challenges of living with a specific disease with staff from FDA’s medical products centers: Center for Drug Evaluation and Research,…